DNA photophysics
Modern research shows that quantum coherent effects play a decisive role in the successful functioning of a number of natural systems [1]. Nitrogenous bases in the NC spirals (Fig. 1) are ordered in the same way as pigments in photosynthetic systems.
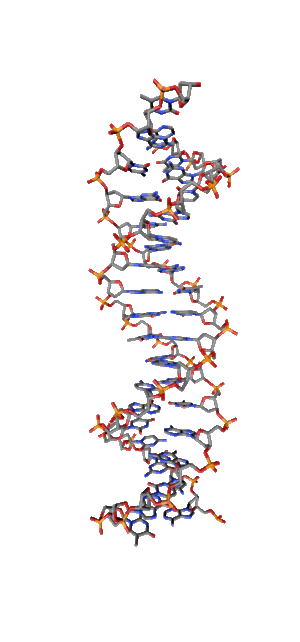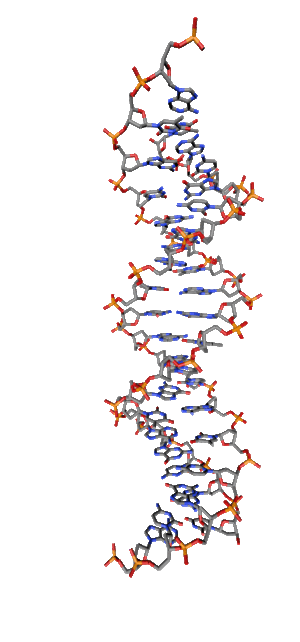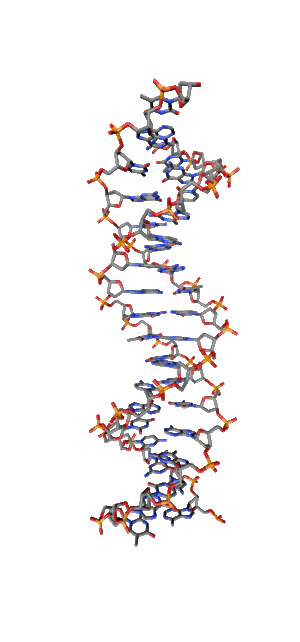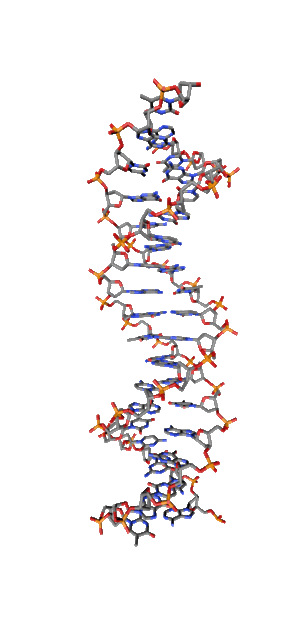
Fig. 1. Schematic structure of the DNA double helix.
Just like in photo system antennas, photon absorption in NC has an excitonic (coherent) character. exciton ordered structure in delocalization of chromophore means, those. collective excitation, which ensures the transport of electronic excitation energy, in the same way as in natural systems of photosynthesis. The DNA helix, as an example of a natural system with a π – π stacking interaction, is widely regarded as a structure in which effective transfer of charge [2, 3] and excitation energy [4] is possible. Exciton and charge transport in NC is of great importance, first of all, for understanding the mechanisms and pathways of photochemical reactions in DNA [5,6], leading to photochemical damage, mutagenesis, and carcinogenesis [7,8]. Direct absorption of UVB photons by DNA in the spectral range of 290-320 nm of solar radiation near the earth's surface is responsible for the formation of cyclobutane pyrimidine dimers (CPD), which cause skin cancer.
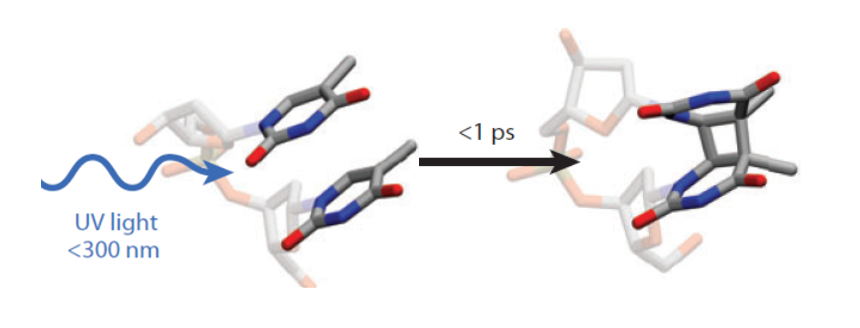
Fig. 2. Formation of a CPD dimer in a DNA helix in a time less than 1 ps after absorption of a photon (adapted from [4]).
Some conformations found in curved DNA structures show a significant redshift of long-wavelength electronic transitions of an excitonic nature, which increases the absorption efficiency of solar UV radiation in such structures, the intensity of which becomes significant in the region> 290 nm, where the absorption of canonical forms DNA drops sharply. We have shown that such structures can be so-called hot spots for photochemical reactions in DNA (RR Ramazanov, DA Maksimov, AI Kononov. Noncanonical Stacking Geometries of Nucleobases as a Preferred Target for Solar Radiation. J. Am. Chem. Soc. 2015, 137 (36), 11656-11665. [DOI link] ).
We study photoprocesses in DNA as a result of cell interaction with UV solar radiation. For example, this figure shows the processes occurring in the structure of the i-motif of DNA:
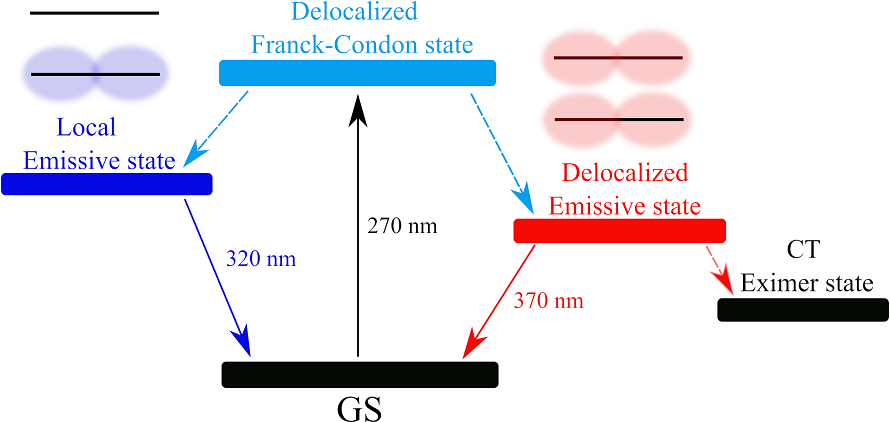
Excited states and the fastest photoprocesses in the i-motif of DNA (ZV Reveguk, EV Khoroshilov, AV Sharkov, VA Pomogaev, AA Buglak, AN Tarnovsky, AI Kononov, Exciton Absorption and Luminescence in i-Motif DNA , Sci. Rep. 2019; 9: 15988. [DOI link]).
We also study the processes and mechanisms of energy migration in DNA complexes with various acceptors. For example, a silver cluster can act as a desensitizer that localizes the UV excitation energy. The rapid transfer of energy from a DNA segment to a cluster (Fig. 3) can neutralize the negative effects of UV radiation and protect DNA and cells from the carcinogenic effects of UV radiation.
 |
Fig. 3. Nucleic Acids Research magazine cover with a picture from our article. Cover: DNA works as antenna transferring excitation energy to metal cluster.For more information see article I. L. Volkov, Z. V. Reveguk, P. Yu. Serdobintsev, R. R. Ramazanov, A. I. Kononov. DNA as UV light-harvesting antenna, Nucleic Acids Res., 2018, 46 (7), 3543-3551. [DOI link] ON COVER |
- M. Arndt, T. Juffmann, and V. Vedral, Quantum physics meets biology. HFSP Journal Vol. 3, No. 6, December 2009, 386–400.
- Slinker, J. D., Muren, N. B., Renfrew, S. E. & Barton, J. K. DNA charge transport over 34 nm. Nat Chem 3, 228-233, (2011).
- Livshits, G. I. et al. Long-range charge transport in single G-quadruplex DNA molecules. Nat Nano advance online publication, (2014).
- Middleton, C. T., de La Harpe, K., Su, C., Law, Y. K., Crespo-Hernández, C. E. & Kohler, B. DNA Excited-state dynamics: from single bases to the double helix. Annu. Rev. Phys. Chem. 60, 217-239, (2009).
- Cadet, J., Mouret, S., Ravanat, J.-L. & Douki, T. Photoinduced damage to cellular DNA: direct and photosensitized reactions. Photochem. Photobiol. 88, 1048-1065, (2012).
- Wurtmann, E. J .; Wolin, S. L. Crit. Rev. Biochem. Mol. Biol. 2009, 44, 34.
- de Gruijl, F. R. Skin cancer and solar UV radiation. Eur. J. Cancer 35, 2003-2009, (1999).
- Pfeifer, G. P., You, Y.-H. & Besaratinia, A. Mutations induced by ultraviolet light. Mutat. Res., Fundam. Mol. Mech. Mutagen. 571, 19-31, (2005).
- Brash, D. E. & Haseltine, W. A. UV-induced mutation hotspots occur at DNA damage hotspots. Nature 298, 189-192, (1982).
- Baverstock, K. F. & Cundall, R. B. Solitons and energy transfer in DNA. Nature 332, 312-313, (1988).

 English (United Kingdom)
English (United Kingdom)  Russian (Russia)
Russian (Russia)